The noble gases have full valence electron shells. Valence electrons are the outermost electrons of an atom and are normally the only electrons that participate in chemical bonding. Atoms with full valence electron shells are extremely stable and therefore do not tend to form chemical bonds and have little tendency to gain or lose electrons. Noble gases exist as individual atoms rather than compounds which can be termed as being ‘monatomic’. Besides being monatomic, they are colorless, odorless, and tasteless as well. Noble Gas Electron Configuration. The distribution of electrons in respective atomic orbits of the noble gases is called noble gas configuration.
Why do all of the noble gases have electron affinities greater than zero?
1 Answer
All Noble Gases Have Valence Electrons
All of the Noble Gases actually have electron affinities of less than or equal to 0.
Explanation:
This is because all of the Noble Gases have complete valence electron shells. Power simulator for mac. They have a complete set of 8 electrons.
Think of 8 as being the best car that everyone wants. Most elements 'want' to have a complete electron shell with 8 electrons. Since the Noble Gases already have that 'perfect status' then they have an affinity of 0.
Affinity is the change in energy of the atom when an electron is added. Noble Gases are at the perfect number of 8 electrons. They don't 'want' anymore electrons, so there's zero change in the energy of the atom.
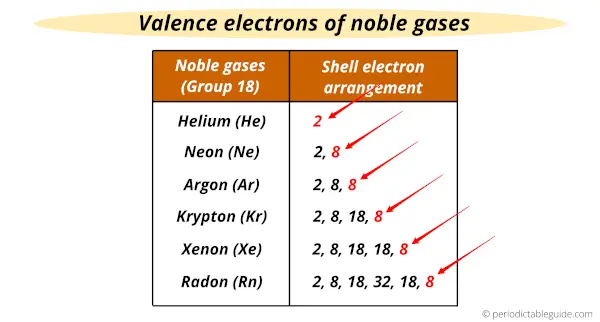
p.s. This is also why they are the least reactive elements on the table.
Related topic

Noble Gases Periodic Table
Related questions
